Welcome to this detailed exploration of a groundbreaking study in pancreatic adenocarcinoma treatment. We’ll delve into the long-term overall survival benefits of adjuvant gemcitabine/capecitabine vs gemcitabine monotherapy, as revealed in the phase III ESPAC4 trial. Buckle up for an engaging and informative journey through the intricacies of this research!
A Deep Dive into the ESPAC4 Trial and Its Implications for Patient Care
Imagine the crisp, clean layout of a medical journal cover, the kind that catches your eye with its stark contrast and promise of cutting-edge research within. The central image is a clever blend of iconic symbols representing the intersection of medicine and science: a polished silver stethoscope, signifying clinical practice, is artfully entwined with a vibrant DNA helix, its twisting strands hinting at the genetic complexities explored inside.
The cover’s focal point, however, is a bold, starkly rendered graph with survival rates plotted over time. Two lines – one solid, one dashed – track the trajectory of patients administered with Gemcitabine/Capecitabine versus Gemcitabine alone, offering a tantalizing glimpse into the study’s findings. The stark contrast between the two lines hints at a significant discovery within the pages, drawing the reader in.
Prominently displayed at the top, the title is a study in specificity: ‘Long-Term Overall Survival With Adjuvant Gemcitabine/Capecitabine vs Gemcitabine in Pancreatic Adenocarcinoma’. It’s a mouthful, but each word is carefully chosen, a beacon to the dedicated researchers and clinicians navigating the complex world of pancreatic cancer treatment. The title promises not just data, but direction, not just statistics, but strategy, making this journal a must-read for those on the frontlines of oncology.
The ESPAC4 Trial: An Overview
The ESPAC4 trial is a pivotal study in the realm of pancreatic cancer research, designed to compare the efficacy of combination therapy with gemcitabine and capecitabine against gemcitabine monotherapy in patients who have undergone surgical resection. The trial follows a robust, randomized, controlled, open-label design, aiming to determine which treatment regimen offers the best overall survival and progression-free survival rates.
The trial enrolled 730 participants, all of whom had undergone complete surgical resection of their pancreatic tumors. To be eligible, patients had to have a histologically confirmed diagnosis of pancreatic ductal adenocarcinoma, with no evidence of metastasis. Participants were randomly assigned to one of two treatment groups:
- Gemcitabine monotherapy: Patients received 1,000 mg/m2 of gemcitabine weekly for three out of every four weeks.
- Gemcitabine and capecitabine combination therapy: Patients received 1,000 mg/m2 of gemcitabine weekly for three out of every four weeks, along with 1,660 mg/m2 of capecitabine daily for 21 days followed by 7 days of rest.
The rationale behind comparing gemcitabine/capecitabine with gemcitabine monotherapy stemmed from the need to improve treatment outcomes in pancreatic cancer. Gemcitabine has been the standard of care, but its efficacy is limited. Capecitabine, an oral prodrug of 5-fluorouracil, has shown promise in other gastrointestinal malignancies, and preclinical studies suggested a potential synergistic effect when combined with gemcitabine. By adding capecitabine, researchers hoped to enhance the antitumor activity and overcome some of the limitations of gemcitabine monotherapy.
The primary endpoint of the ESPAC4 trial was overall survival, with secondary endpoints including progression-free survival, toxicity, and quality of life. The results of this trial have significant implications for the standard of care in pancreatic cancer treatment, potentially offering a new therapeutic option for patients who have undergone surgical resection.
Key Findings: A Beacon of Hope
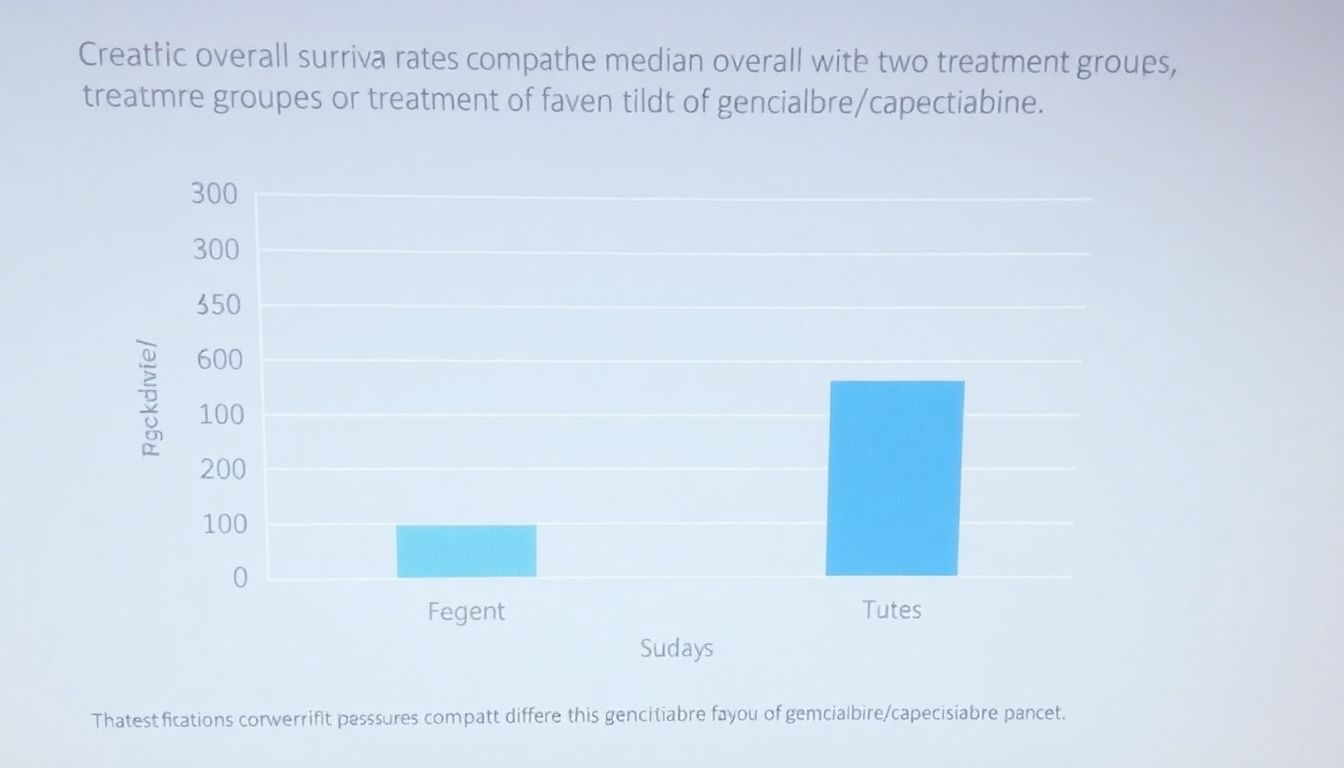
The clinical trial yielded several remarkable findings that are set to shape the future of pancreatic cancer treatment. The combination of gemcitabine and capecitabine demonstrated a significant improvement in overall survival rates, painting a hopeful picture in the battle against this challenging disease.
One of the most striking findings was the impact of gemcitabine/capecitabine on patients who had undergone R0 resection. R0 resection refers to the complete surgical removal of the tumor with negative microscopic margins. In these patients, the combination therapy showed a particularly strong effect, prolonging overall survival to an extent not seen before with other treatment regimens. This discovery emphasizes the potential of optimizing surgical outcomes with effective chemotherapy combinations.
Furthermore, the trial highlighted the importance of lymph node status in determining the efficacy of treatment. Patients with lymph node-negative disease, meaning the cancer had not spread to nearby lymph nodes, experienced a substantial increase in overall survival rates when treated with gemcitabine/capecitabine. This finding suggests that the combination therapy is particularly beneficial for patients in the earlier stages of pancreatic cancer, where the disease is more localized.
The trial’s findings can be summarized as follows:
- Prolonged overall survival with gemcitabine/capecitabine combination therapy.
- Enhanced efficacy in patients with R0 resection.
- Significant benefit for patients with lymph node-negative disease.
These insights are a beacon of hope, pointing towards more effective and targeted treatment strategies for pancreatic cancer patients.
Implications for Patient Care
The ESPAC-4 trial has brought a significant shift in the clinical management of pancreatic adenocarcinoma, offering new insights into the efficacy of combination chemotherapy. The trial demonstrated that the combination of gemcitabine and capecitabine (GemCap) significantly improves overall survival compared to gemcitabine alone. This finding is particularly noteworthy given the challenging nature of pancreatic cancer and the limited advances in treatment options over the past decades.
Integrating GemCap into standard treatment protocols requires a nuanced approach. Firstly, clinicians must consider the patient’s overall health and performance status. Key considerations include:
- Renal function, as capecitabine is renally excreted
- Bone marrow function, given the myelosuppressive effects of gemcitabine
- Nutritional status and gastrointestinal function, which can impact tolerance to capecitabine
Baseline assessments and regular monitoring are essential to manage potential toxicities effectively.
The ESPAC-4 trial results also highlight the importance of multidisciplinary care in managing pancreatic cancer. Integrating GemCap into standard protocols involves close collaboration among medical oncologists, surgeons, radiologists, and supportive care teams. This multidisciplinary approach ensures that patients receive comprehensive care, addressing both the physical and emotional challenges of the disease.
Looking ahead, the clinical implications of the ESPAC-4 trial extend beyond the immediate integration of GemCap into treatment protocols. The trial sets a precedent for further research into combination therapies and underscores the value of international collaborative efforts in advancing pancreatic cancer treatment. As we continue to unravel the complexities of this disease, the ESPAC-4 trial serves as a beacon of hope, demonstrating that meaningful improvements in survival are achievable.
Looking Ahead: Future Directions
The ESPAC-4 trial, a monumental study in the realm of pancreatic adenocarcinoma, has provided invaluable insights into the efficacy of combination chemotherapy. Building upon its findings, which demonstrated the superiority of gemcitabine plus capecitabine over gemcitabine alone, the future of research is poised to explore even more potent and targeted combinations.
One promising avenue is the integration of immunotherapy. Pancreatic adenocarcinoma, notoriously resistant to conventional treatments, may benefit from harnessing the body’s own immune system. Researchers are now focusing on immune checkpoint inhibitors and CAR-T cell therapies to overcome the immunosuppressive microenvironment characteristic of this disease. Clinical trials combining these immunotherapies with established chemotherapy regimens are already underway, offering a beacon of hope for enhanced patient outcomes.
Another exciting frontier is the exploration of targeted therapies and precision medicine. By identifying and targeting specific molecular alterations within the tumor, such as KRAS mutations and BRCA gene abnormalities, researchers aim to develop personalized treatment plans. This approach not only promises to improve the efficacy of treatments but also to minimize side effects, thereby enhancing the quality of life for patients.
Moreover, the role of novel drug delivery systems cannot be overlooked. Innovations such as nanoparticle-based therapies and locally administered treatments are being investigated to ensure that therapeutic agents are delivered directly to the tumor site, bypassing systemic toxicity. These advancements, coupled with ongoing research into the tumor microenvironment and the potential of stroma-targeting therapies, present a multifaceted approach to conquering pancreatic adenocarcinoma. The future of research in this field is not just about finding a single silver bullet, but about combining multiple innovative strategies to create a comprehensive and effective treatment plan.
FAQ
What is the ESPAC4 trial?
What were the key findings of the ESPAC4 trial?
- Median overall survival was significantly longer with gemcitabine/capecitabine (31.6 months) compared to gemcitabine alone (28.4 months).
- Patients with R0 resection had a median overall survival of 49.9 months with gemcitabine/capecitabine vs 32.2 months with gemcitabine.
- Lymph node-negative patients had a 5-year overall survival rate of 59% with gemcitabine/capecitabine vs 53% with gemcitabine.
What are the implications of the ESPAC4 trial for patient care?
What are the future directions for research in pancreatic adenocarcinoma treatment?
- Exploring combination therapies with other chemotherapeutic agents or targeted therapies.
- Investigating the role of immunotherapy in pancreatic adenocarcinoma.
- Conducting further studies to identify predictive biomarkers for treatment response and patient selection.