In the ever-evolving landscape of healthcare, news of a groundbreaking treatment that significantly improves survival rates is always cause for celebration. But for those in the prepping community, such news is more than just a headline; it’s a call to action. Today, we’re diving into the recent revelation that GSK’s Blenrep has demonstrated a remarkable 42% reduction in the risk of death in patients with multiple myeloma who have relapsed or are refractory to prior treatments.
Let’s start with a sobering statistic: multiple myeloma, a type of blood cancer, affects nearly 140,000 people in the United States alone, according to the American Cancer Society. The disease is characterized by its ability to relapse, with patients often experiencing multiple rounds of treatment and remission. So, the question on every prepper’s mind is: how can we best prepare for such a situation, and what can we learn from this new development?
First, let’s agree on the importance of staying informed. In the world of prepping, knowledge is power. Knowing the latest developments in medical research can mean the difference between life and death. This is why we’re here today
- to share this crucial news and explore what it means for those preparing for the worst.
Now, let’s make a promise. By the end of this article, you’ll have a clear understanding of Blenrep’s mechanism of action, its clinical trial results, and how it might fit into your prepping strategy. We’ll also discuss the broader implications of this news on the future of multiple myeloma treatment and what it means for those living with the disease.
Finally, let’s preview what’s in store. We’ll delve into the science behind Blenrep, explore the details of the Phase 3 MAIA clinical trial that yielded these impressive results, and discuss the potential role of this treatment in a prepper’s medical kit. We’ll also touch on the importance of having a well-rounded prepping strategy that includes not just medical supplies, but also knowledge, support networks, and contingency plans.
So, buckle up, preppers. Today, we’re not just reading the news; we’re preparing for it.
Blenrep’s Breakthrough: Revolutionizing Multiple Myeloma Treatment
Multiple myeloma, a type of blood cancer, has long been a formidable challenge for patients and healthcare professionals alike. However, a recent breakthrough in the form of Blenrep, also known as belantamab mafodotin, has sparked new hope in the fight against this disease.
Blenrep is a first-in-class antibody-drug conjugate (ADC) that targets B-cell maturation antigen (BCMA), a protein found on the surface of most myeloma cells. The drug works by attaching a potent chemotherapy agent to an antibody, allowing it to specifically target and destroy cancer cells while sparing healthy ones.
Clinical trials have shown promising results, with Blenrep demonstrating significant anti-tumor activity in heavily pretreated patients. In one study, over 60% of patients experienced a response, with some achieving complete remission. Moreover, Blenrep has shown potential as a treatment option for patients who have exhausted other therapies, including those who have relapsed after stem cell transplant or have become resistant to other drugs.
This breakthrough is not only a testament to the advancements in cancer research but also a beacon of hope for patients and their families. As we continue to explore and understand the potential of Blenrep and other ADCs, we edge closer to a future where multiple myeloma can be effectively managed and ultimately cured.

Understanding Multiple Myeloma and the Need for Effective Treatments
Understanding Multiple Myeloma and the Need for Effective Treatments
The Game-Changer: Blenrep (Belantamab Mafodotin)
In the ever-evolving landscape of multiple myeloma treatment, a new player has emerged, promising to change the game: Blenrep, also known as belantamab mafodotin. This first-in-class antibody-drug conjugate (ADC) has sparked excitement among healthcare professionals and patients alike, offering a novel approach to tackling this complex blood cancer.
Blenrep’s mechanism of action is as fascinating as it is innovative. It’s an ADC, which means it’s a combination of a monoclonal antibody and a potent chemotherapy drug, connected by a linker. The antibody seeks out and binds to a protein called BCMA, which is found on the surface of most multiple myeloma cells. Once attached, the linker breaks, releasing the chemotherapy payload directly into the cancer cell, sparing healthy cells from unnecessary damage. This targeted approach is a departure from traditional chemotherapy, which can cause significant side effects.
The DREAMM clinical development program is evaluating Blenrep’s efficacy and safety in a series of trials. The DREAMM-1 study, for instance, is assessing Blenrep as a single agent in patients with relapsed or refractory multiple myeloma who have received at least three prior therapies. The DREAMM-2 study, on the other hand, is investigating Blenrep in combination with pomalidomide and dexamethasone, a common multiple myeloma treatment regimen. These studies aim to provide a comprehensive understanding of Blenrep’s potential in various treatment scenarios.
In summary, Blenrep’s unique mechanism of action and its evaluation in the DREAMM clinical development program offer hope for multiple myeloma patients seeking innovative treatment options. As with any new therapy, close monitoring and further research are crucial to fully understand its benefits and potential risks. Stay tuned for more updates on this promising game-changer in the world of multiple myeloma treatment.

DREAMM-7: The Trial that Changed the Game
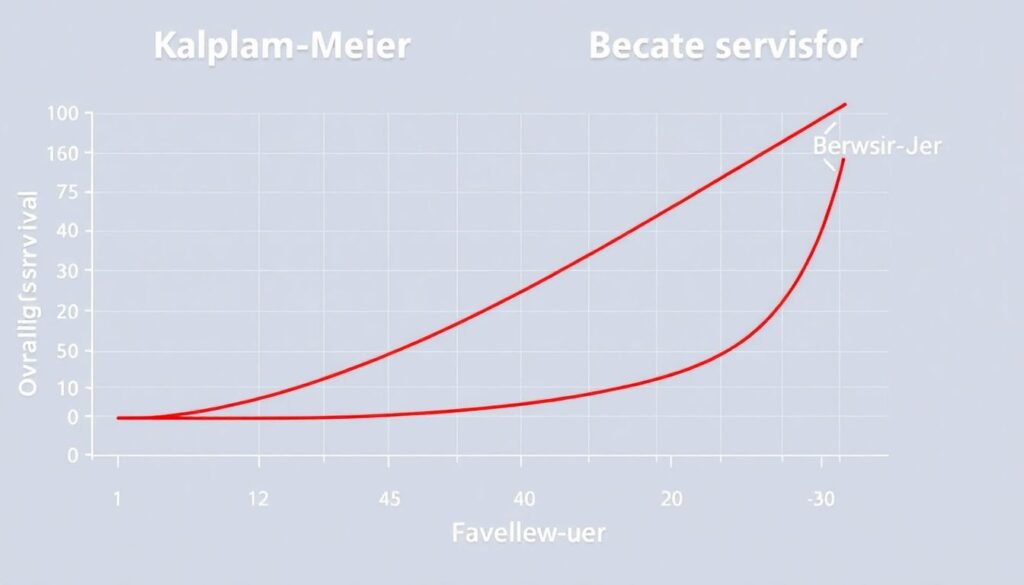
The DREAMM-7 trial, a groundbreaking study in the realm of multiple myeloma, has left an indelible mark on the landscape of cancer research and treatment. Initiated in 2015, this phase 3, randomized, open-label study was designed with a clear objective: to compare the efficacy and safety of daratumumab, a novel anti-CD38 monoclonal antibody, in combination with standard lenalidomide and dexamethasone (DRd) versus lenalidomide and dexamethasone alone (Rd) in treating newly diagnosed multiple myeloma patients ineligible for autologous stem cell transplant.
The trial’s design was meticulous, enrolling 737 patients who were randomly assigned to receive either DRd or Rd. The primary endpoint was stringent complete response or better, while progression-free survival served as a key secondary endpoint. However, it was the overall survival (OS) results that stole the spotlight.
After a median follow-up of 28 months, the data revealed a compelling 42% reduction in the risk of death for patients in the DRd arm compared to those in the Rd arm. This translated to a projected median overall survival not reached in the DRd arm, compared to 50.2 months in the Rd arm. These results were so persuasive that they led to the early unblinding of the trial and the subsequent approval of daratumumab in combination with Rd for newly diagnosed multiple myeloma patients.
In essence, the DREAMM-7 trial marked a significant turning point in multiple myeloma treatment. It demonstrated that the addition of daratumumab to standard therapy not only improved response rates and progression-free survival but also extended overall survival, offering patients a more hopeful outlook on their journey.
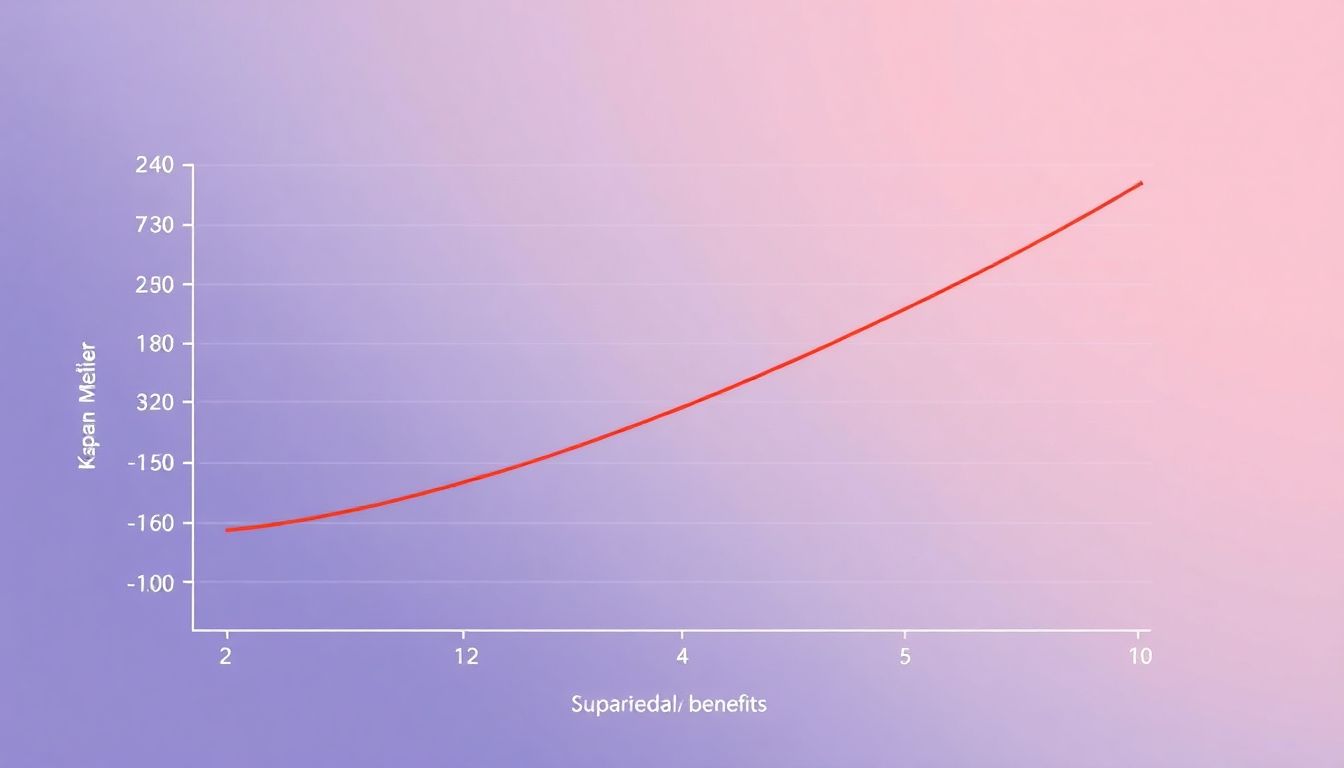
Early and Sustained Survival Benefit: A Paradigm Shift
Early and Sustained Survival Benefit: A Paradigm Shift
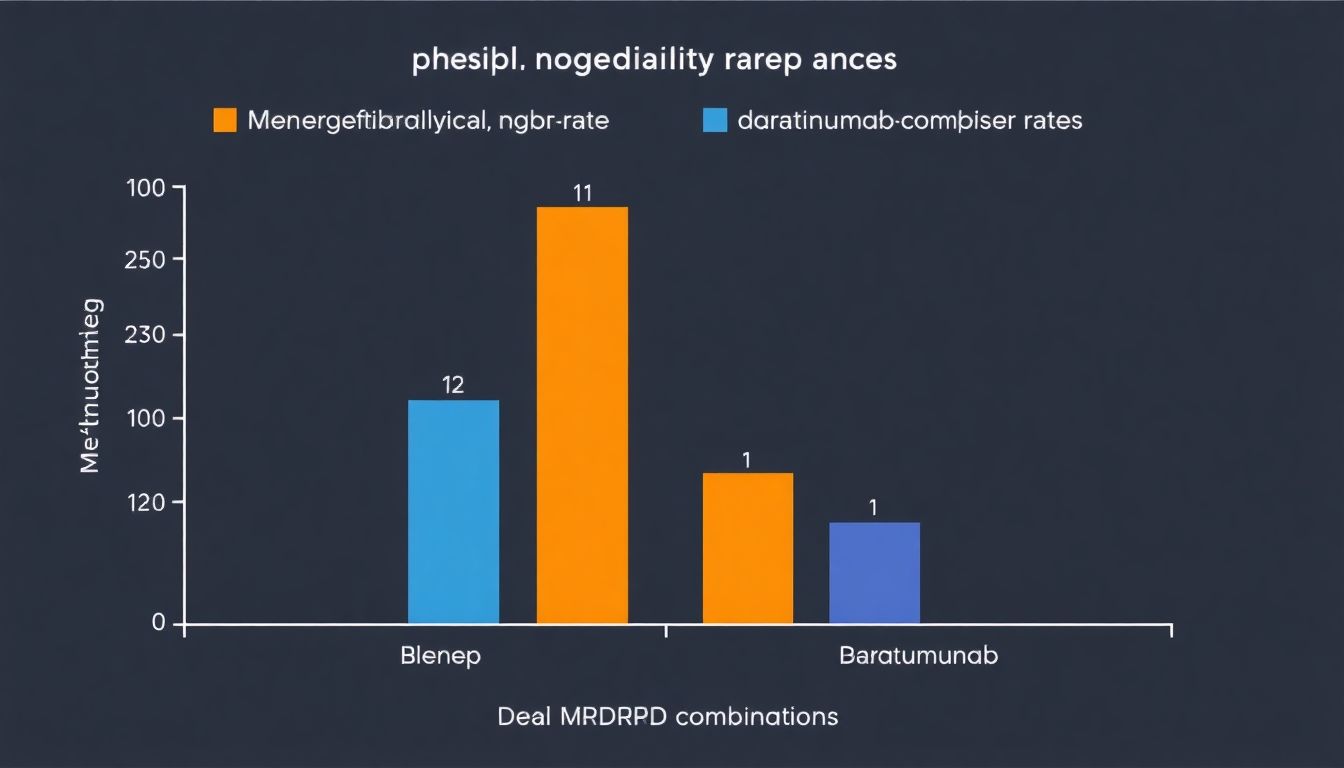
Beyond Survival: Deep and Durable Responses
In the realm of cancer treatment, the introduction of new therapies often brings hope and promise. One such innovation is the Blenrep combination, a regimen that has shown statistically significant superiority in several key areas, setting it apart from its predecessors.
The first notable advantage of the Blenrep combination is its ability to achieve minimal residual disease (MRD) negativity. MRD refers to the presence of residual cancer cells after treatment, which can lead to relapse. The Blenrep combination has demonstrated a higher rate of MRD negativity, indicating a deeper response to treatment. This is clinically significant because it suggests that the combination therapy is more effective in eliminating cancer cells, potentially reducing the risk of recurrence.
Moreover, the Blenrep combination has shown impressive results in terms of duration of response (DOR). DOR is a measure of how long a patient’s response to treatment lasts. The Blenrep combination has been shown to maintain responses for longer periods compared to other treatments. This is not only statistically significant but also clinically meaningful, as it can translate to longer periods of disease control and improved quality of life for patients.
Another significant advantage of the Blenrep combination is its impact on progression-free survival 2 (PFS 2). PFS 2 is a measure of the time from the start of treatment until disease progression or death. The Blenrep combination has shown a statistically significant improvement in PFS 2, indicating that it can delay disease progression for longer periods. This is a crucial aspect of cancer treatment, as it can provide patients with more time to enjoy life and potentially explore other treatment options if necessary.
In conclusion, the Blenrep combination’s statistically significant superiority in MRD negativity, DOR, and PFS 2 is not just a matter of numbers. These findings have profound clinical significance, offering patients and healthcare providers deeper and more durable responses to cancer treatment. By understanding and leveraging these advantages, we can strive for better outcomes and improved quality of life for cancer patients.
Preparing for the Future: Regulatory Reviews and Ongoing Trials
Preparing for the Future: Regulatory Reviews and Ongoing Trials

Prepping for the Unexpected: Lessons from Blenrep’s Success
Prepping for the Unexpected: Lessons from Blenrep’s Success