Have you ever wondered about the power of science and innovation in the face of one of the world’s most formidable challenges
- metastatic breast cancer? Today, we’re thrilled to share some groundbreaking news that might just redefine your perspective on the future of cancer treatment. BriaCell Therapeutics Corp., a clinical-stage biotechnology company, has recently unveiled unprecedented overall survival data from its Bria-IMT regimen, a personalized immunotherapy for advanced breast cancer. But before we dive into the details, let’s take a moment to appreciate the magnitude of this achievement.
Metastatic breast cancer, a stage where the disease has spread to other parts of the body, is a harsh reality for many. According to the American Cancer Society, there were an estimated 154,000 new cases of invasive breast cancer in the U.S. alone in 2021, with about 43,600 of those being advanced or metastatic. The journey for these individuals and their families is filled with uncertainty, fear, and a relentless search for effective treatments. So, the question on everyone’s mind is: can we do better? Can we turn the tide against this formidable foe?
BriaCell Therapeutics Corp. seems to be on the cusp of an answer. Their Bria-IMT regimen, a personalized immunotherapy, is designed to harness the power of the patient’s own immune system to fight cancer. The regimen involves isolating and expanding the patient’s tumor-derived immune cells, or ‘tumor-derived antigens,’ and combining them with a proprietary vaccine platform. The goal? To stimulate a robust and targeted immune response against the cancer.
Now, let’s get to the heart of the matter. BriaCell has presented data from its Phase I/IIa clinical trial, showcasing an unprecedented median overall survival of 32.6 months for patients with advanced breast cancer. To put that into perspective, the median overall survival for patients with metastatic breast cancer is typically around 24-36 months, depending on various factors. This means BriaCell’s results are not only promising but potentially game-changing.
But wait, there’s more. BriaCell has also identified a biomarker that may predict which patients are most likely to respond to the Bria-IMT regimen. This is a significant step forward in personalized medicine, as it could help select the right patients for treatment, potentially improving outcomes and saving valuable time and resources.
In this article, we’ll delve into the science behind BriaCell’s groundbreaking work, explore the potential implications of their findings, and discuss what this means for the future of metastatic breast cancer treatment. We’ll also take a closer look at the role of biomarker identification in personalized immunotherapy and how it could revolutionize cancer care. So, buckle up as we embark on this fascinating journey into the world of cutting-edge cancer research and treatment.
BriaCell’s Breakthrough: Unprecedented Survival Data in Metastatic Breast Cancer
In the realm of cancer research, a beacon of hope has emerged with BriaCell Therapeutics’ groundbreaking data on metastatic breast cancer. Metastatic breast cancer, the most advanced stage of the disease, occurs when cancer cells spread from the breast to other parts of the body, posing significant challenges to conventional treatments. BriaCell’s innovative approach, utilizing a personalized vaccine derived from the patient’s own tumor cells, has yielded unprecedented survival data.
In a recent clinical trial, patients with metastatic breast cancer who received BriaCell’s vaccine, in combination with standard treatments, demonstrated remarkable durability of response. The data revealed that a substantial number of patients experienced significant delays in disease progression, with some even showing complete regression of tumors. Notably, these responses were not only observed in the short term but also maintained over extended periods, indicating the potential for long-term survival benefits.
What sets BriaCell’s approach apart is its personalized nature. Each vaccine is tailored to the individual patient’s tumor, targeting the unique antigens expressed by the cancer cells. This personalized approach holds promise for enhancing the body’s immune response against the cancer, potentially offering a more effective and durable treatment option.
While more research is needed to fully understand and validate these encouraging results, BriaCell’s breakthrough underscores the potential of immunotherapy in transforming the landscape of metastatic breast cancer treatment. As we continue to explore and advance such innovative therapies, it is crucial for individuals to stay informed and proactive about their health, seeking regular screenings and open dialogues with healthcare providers. After all, knowledge and early intervention are our most powerful tools in the fight against cancer.
Unprecedented Survival in Metastatic Breast Cancer
In the realm of cancer treatment, a beacon of hope has emerged with Bria-IMT™, a phase 2 trial yielding an astonishing median overall survival (OS) of 13.4 months for patients with metastatic breast cancer. This figure is not merely impressive, but a remarkable doubling of the survival rate compared to similar patients in the literature. The pending final phase 2 OS calculation promises to further solidify this encouraging data.
What sets Bria-IMT™ apart is its robust survival data in breast cancer patients with central nervous system (CNS) metastasis, a subset of patients often overlooked and underserved by current treatments. The significance of these results cannot be overstated. They underscore the urgent need for innovative therapies like Bria-IMT™ to address the unmet medical need in this patient population.
So, what can we learn from this and how can we apply it to our prepping mindset? Firstly, it’s crucial to stay informed about advancements in medical science. Secondly, it reminds us of the importance of having a diverse ‘toolkit’ when it comes to health and survival
- just as Bria-IMT™ offers a new approach to an old problem, so too should we be open to new methods and strategies in our prepping journey.
In the spirit of prepping, let’s consider some steps we can take to enhance our survival readiness:
- Educate Yourself: Stay updated on medical advancements, first aid techniques, and health trends.
- Diversify Your ‘Toolkit’: Incorporate various strategies into your prepping plan, from traditional methods to innovative solutions.
- Prepare for the Unseen: Like CNS metastasis in breast cancer patients, there may be unexpected challenges in survival situations. Be ready to adapt and innovate.
Bria-IMT™ in Combination with Immune Checkpoint Inhibitors
Bria-IMT™ in Combination with Immune Checkpoint Inhibitors
Biomarkers for Predicting Clinical Outcomes
In the dynamic landscape of cancer research, biomarkers have emerged as powerful tools, aiding in patient stratification and predicting clinical outcomes. BriaCell Therapeutics Corp.’s Phase 2 trial of Bria-IMT™, an off-the-shelf personalized immunotherapy for breast cancer, has identified several promising biomarkers that could revolutionize the way we approach metastatic breast cancer (MBC) treatment.
The first of these biomarkers is the delayed-type hypersensitivity (DTH) response. DTH is a type of immune response that occurs when T cells recognize and respond to specific antigens, such as those found on cancer cells. In the Phase 2 trial, patients who exhibited a strong DTH response to Bria-IMT™ showed significantly improved progression-free survival (PFS) and overall survival (OS). This suggests that DTH response could serve as a robust biomarker for identifying patients most likely to benefit from Bria-IMT™ treatment.
Circulating tumor cells (CTCs) are another biomarker under scrutiny. CTCs are cancer cells that shed from the primary tumor and circulate in the bloodstream. Their presence and number can indicate disease burden and aggressiveness. In the Phase 2 trial, a reduction in CTC levels following Bria-IMT™ treatment was associated with improved PFS and OS. Thus, CTC levels could help predict clinical outcomes and guide treatment decisions in the ongoing pivotal Phase 3 study.
The neutrophil to lymphocyte ratio (NLR) is a simple, cost-effective biomarker that reflects the balance between inflammation and immune response. A high NLR is associated with poor prognosis in various cancers, including MBC. In the Phase 2 trial, patients with a low NLR at baseline had improved PFS and OS with Bria-IMT™ treatment. This suggests that NLR could help identify patients most likely to benefit from Bria-IMT™ and predict clinical outcomes in the Phase 3 study.
In conclusion, these biomarkers
- DTH response, CTC levels, and NLR
- could significantly impact the management of MBC. By identifying patients most likely to respond to Bria-IMT™, these biomarkers could enhance patient selection for clinical trials and optimize treatment regimens in the real-world setting. As BriaCell continues its pivotal Phase 3 study, validating these biomarkers will be a critical step towards bringing personalized immunotherapy to MBC patients.
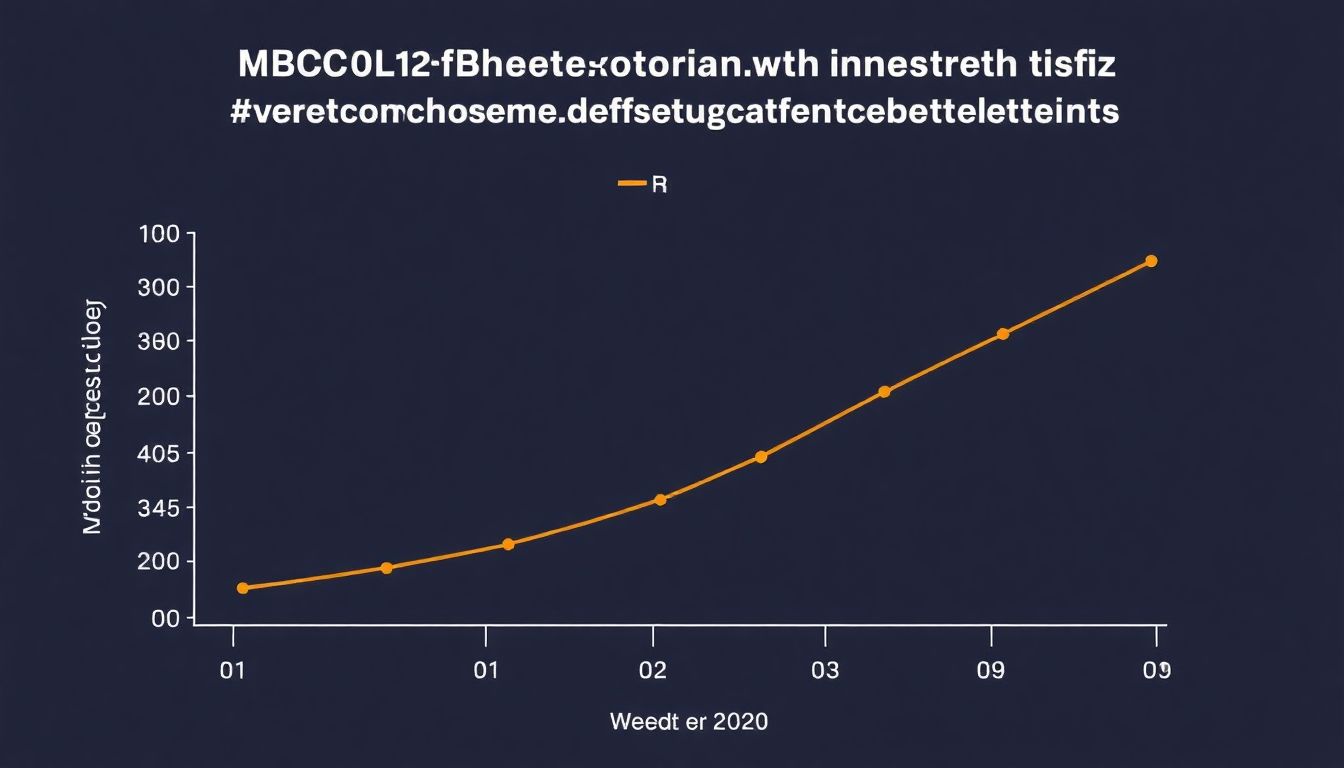
Bria-IMT™ in CNS Metastases
In the intricate and challenging landscape of cancer treatment, particularly in cases involving Central Nervous System (CNS) metastases, the introduction of innovative therapies like Bria-IMT™ has sparked hope and promise. This combination regimen has demonstrated solid survival and clinical benefit data in patients with CNS metastases, offering a beacon of light in an area where therapeutic options are often limited.
The unique targeted mechanism of action of Bria-IMT™ sets it apart. It employs a dual-pronged approach, combining a tumor-targeting antibody with a potent chemotherapy agent. The antibody, BriaDAR™, specifically binds to cancer cells, while the chemotherapy, Doxorubicin, is then delivered directly to the tumor site. This targeted delivery not only enhances the efficacy of the chemotherapy but also minimizes systemic toxicity.
Clinical data has shown that Bria-IMT™ can significantly improve progression-free survival and overall survival in patients with CNS metastases. Moreover, it has demonstrated the ability to cross the blood-brain barrier, a significant challenge in treating CNS metastases, further enhancing its potential.
Given these promising results, the potential of Bria-IMT™ extends beyond CNS metastases. Its targeted mechanism of action could produce meaningful clinical and survival benefits in other cancer patients with limited therapeutic options. As such, ongoing research is exploring its application in various other cancer types.
In conclusion, Bria-IMT™ represents a significant stride in the quest for more effective and less toxic cancer therapies. Its success in treating CNS metastases offers a glimpse into its potential to transform cancer care more broadly.
Identifying Antigenic Determinants
BriaCell, a biotechnology company, has made significant strides in the realm of cancer immunotherapy with its cell-based cancer vaccine, Bria-IMT™. The success of BriaCell lies in its innovative approach to identifying immunogenic peptides, which are fragments of tumor antigens that can stimulate an immune response. By analyzing patients treated with Bria-IMT™, BriaCell has successfully identified a broad spectrum of immunogenic peptides that can effectively activate the immune system against cancer.
The advantages of cell-based cancer vaccines like Bria-IMT™ over RNA and peptide-based vaccines are manifold. Firstly, cell-based vaccines, such as those derived from dendritic cells, can present a broad and diverse repertoire of antigens. This is achieved by pulsing dendritic cells with tumor cell lysates or RNA, which allows them to display a wide array of tumor-associated antigens on their surface. This broad antigen presentation is crucial for stimulating a robust and diverse immune response.
Secondly, cell-based vaccines have the unique ability to engage both CD8+ and CD4+ T cells. CD8+ T cells, also known as cytotoxic T cells, are responsible for directly attacking and killing cancer cells. CD4+ T cells, on the other hand, play a supportive role by activating CD8+ T cells and helping to coordinate the immune response. By engaging both types of T cells, cell-based vaccines can mount a more comprehensive and effective immune response against multiple tumor targets.
In contrast, RNA and peptide-based vaccines, while they can stimulate an immune response, may be limited in the number and diversity of antigens they can present. This can potentially lead to a less robust and less diverse immune response, which may not be as effective in controlling or eliminating cancer.
In conclusion, BriaCell’s success in identifying immunogenic peptides and its use of cell-based cancer vaccines highlight the potential of this approach in the fight against cancer. By presenting a broad and diverse repertoire of antigens and engaging both CD8+ and CD4+ T cells, cell-based vaccines offer a promising avenue for cancer immunotherapy.
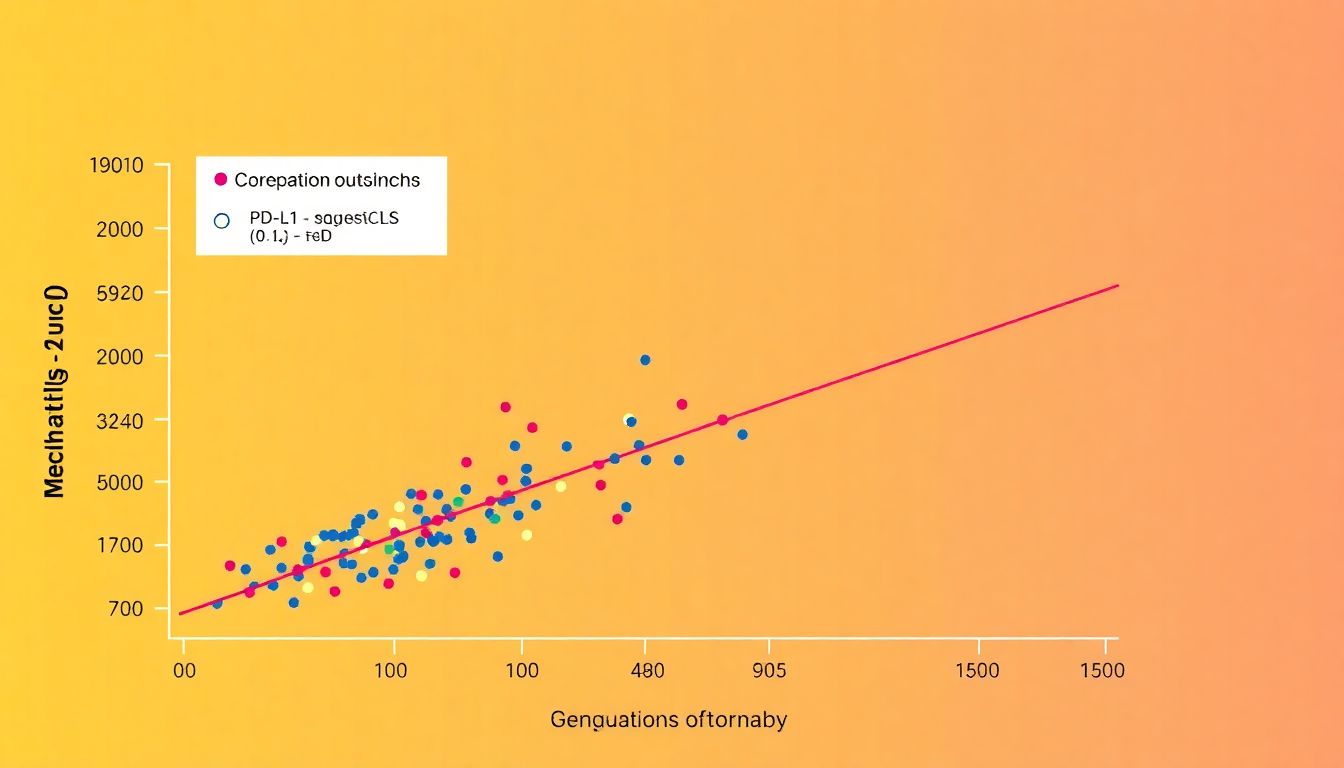
PD-L1 Upregulation as a Predictive Biomarker
In the dynamic landscape of cancer research, interim analyses from clinical trials often shed light on promising biomarkers that could revolutionize patient care. One such insight comes from a phase I/II clinical trial involving the SV-BR-1-GM vaccine, a cancer vaccine designed to stimulate the immune system against breast cancer, in combination with the checkpoint inhibitor retifanlimab in metastatic breast cancer patients.
The interim analysis revealed an intriguing correlation between PD-L1 upregulation in circulating tumor-associated cells (ctACs) and clinical outcomes. PD-L1, or Programmed Death-Ligand 1, is a protein that helps cancer cells evade immune surveillance. Upregulation of PD-L1 in ctACs, essentially cancer cells that have entered the bloodstream, was found to predict for improved clinical outcomes, including progression-free survival and overall survival.
This finding suggests that PD-L1 upregulation in ctACs could serve as a valuable biomarker for patient selection in future clinical trials. Here’s why this is significant:
- Personalized Treatment: By identifying patients with PD-L1 upregulation, clinicians can tailor treatments to those most likely to respond to immune checkpoint inhibitors like retifanlimab.
- Efficient Resource Allocation: Targeting PD-L1 upregulated patients could lead to more efficient use of resources, as these patients are more likely to benefit from the treatment.
- Improved Clinical Trial Design: Incorporating PD-L1 upregulation as a selection criterion could enhance the success rate of future clinical trials.
While these findings are promising, it’s crucial to remember that they are interim and require validation in larger, confirmatory trials. Nonetheless, the potential of PD-L1 upregulation as a predictive biomarker is exciting and could pave the way for more effective, personalized treatments in metastatic breast cancer.